Introducing SafeBreak Vascular
SafeBreak Vascular reduces the number of complications requiring IV restarts by separating when a harmful force is placed on an IV line. When SafeBreak intentionally separates, it has valves that seal both sides of the line, preventing the leaking of medication or blood from the IV tubing. Examples of SafeBreak installed in an IV line, a force being applied to the line, and SafeBreak separating to remove the harmful force and prevent spills from the line are shown below.
Clearance For All Vascular Lines
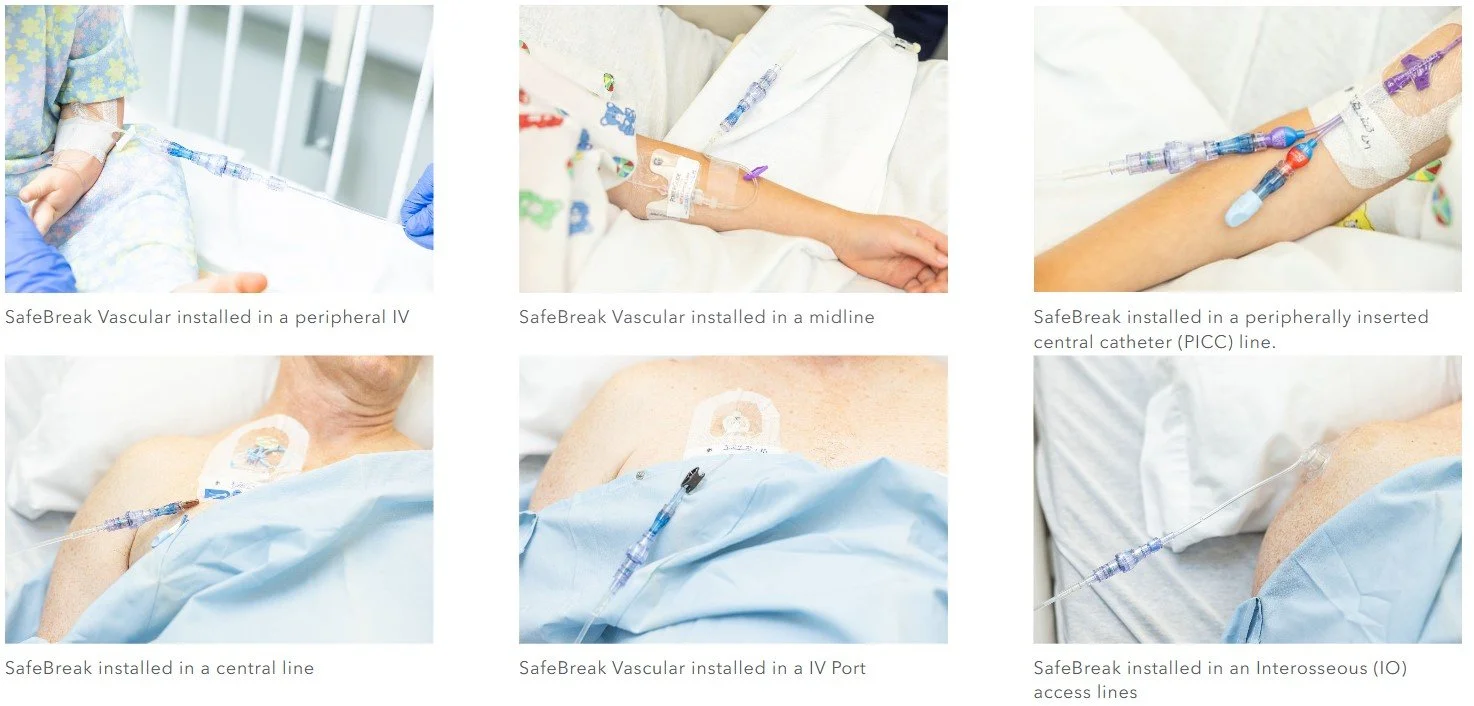
SafeBreak Vascular is the first breakaway device to be cleared on all vascular access lines including peripheral IV catheters, midlines, peripherally inserted central catheters, central venous catheters, IV Ports, and interosseous vascular access devices on patients two weeks and up. This allows SafeBreak Vascular to be installed on both adults and children on every type of IV line.
Clinical Results
A Randomized Controlled Study Showed A 44% Reduction In Peripheral IV Complications Requiring An IV Restart! As a nurse, you know that peripheral IVs fail at an alarming rate - 46% of the time. IV mechanical complications such as phlebitis, infiltration, occlusion, and dislodgement are the main culprits causing these failures. IVs have been around for almost 100 years. Why do we still have such a miserable peripheral IV failure rate?
In a prospective randomized clinical trial conducted at Hartford Hospital (Hartford, CT) in 2020, SafeBreak reduced the number of peripheral IV complications that required an IV restart by 44%. The data showed that 144 patients in the control group had 39 complications requiring an IV restart, while the 143 patients in the SafeBreak group had only 22 complications. “SafeBreak® Vascular is intended to aid in reduction of IV and IO mechanical complications requiring IV and IO replacement.” See SafeBreak Vascular’s Instructions For Use for the complete Indications For Use. To receive the D.I.P.P.E.R. clinical study please contact us, and check out the further information on SfeBreak by clicking the button at the top of the page.